Natalizumab is a monoclonal antibody that is only used in humans with multiple sclerosis. It is somewhat toxic, and we were interested to see if this large monoclonal antibody transfers into human milk. Levels in milk appear low, but I made a mistake in the timing of the study, and should have drawn milk levels several months longer. I need to redo this study and go out to about 120 days. However, at this point, I still think milk levels are subclinical. TWH
Title: Transfer of natalizumab into breast milk in a mother with multiple sclerosis.
Authors: Baker TE, Cooper SD, Kessler L, Hale TW.
J Hum Lact. 2015 May;31(2):233-6.
Abstract:
Natalizumab (Tysabri) is a recombinant humanized antibody to α4-integrin that is approved by the Food and Drug Administration for the treatment of multiple sclerosis (MS) and Crohn disease. This is a case report of a 28-year-old woman with MS who was taking natalizumab (300 mg intravenously infused over 1 hour every 4 weeks) while breastfeeding her 11.5-month-old daughter 3 times a day. Breast milk samples were collected over a 50-day period after the patient's first drug infusion. The average concentration of natalizumab was 0.93 µg/mL/d, and the relative infant dose was 1.74% of the weight-adjusted maternal dose. Transfer of natalizumab into human milk increased over time and with subsequent injections, with the highest concentration of 2.83 µg/mL at day 50 with a relative infant dose of 5.3%. Because these data suggest continued accumulation of natalizumab in milk, and because we cannot provide an accurate assessment of levels of this drug at 24 weeks (steady state), we are unable to determine safety at this time.
Figure 1. Natalizumab Concentration in Breast Milk.
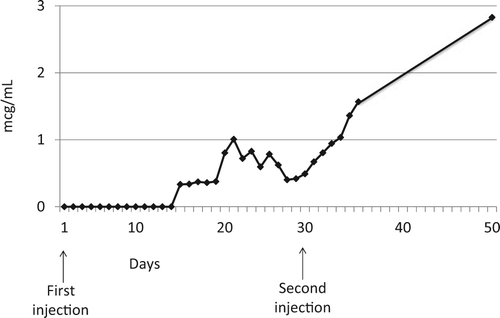
Concentration of natalizumab in breast milk at steady state versus time following 2 intravenous injections of natalizumab (300 mg).
PMID 25586712
