InfantRisk Center: We specialize in lactation research
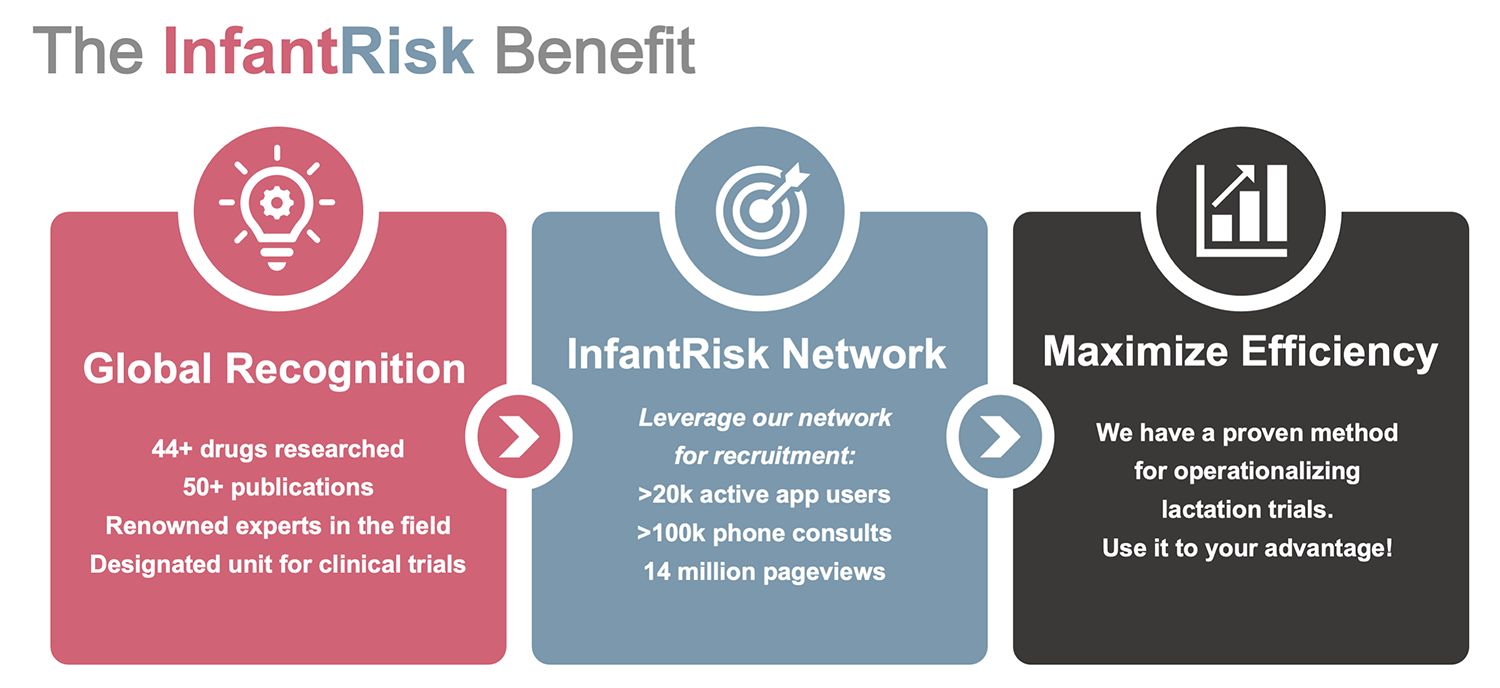
Lactation is a world that often goes unexplored. The InfantRisk Center is committed to analyzing the complexities of medication use while breastfeeding, studying one drug at a time. Our name is recognized worldwide for our expertise in this field. We have successfully analyzed over 44 drugs in breastfeeding women. Each drug requires a unique evaluation for optimized study design.
Considering we only have data on 400 drugs in lactating women, the InfantRisk Center built over 10% of the entire knowledge base in the field. No one does lactation like we do.
Recruitment
Lactation drug studies frequently fail due to their inability to identify potential subjects, terminating after incurring substantial start-up costs. The InfantRisk Center is uniquely positioned to overcome this obstacle as a trusted resource of medication information during breastfeeding. Our apps and call center directly connect us to the breastfeeding women taking medications. We have over 20,000 monthly active users on our apps, and take 3x more lactational exposure calls than the Poison Control Centers nationwide. This extensive network allows us to more efficiently recruit, and complete, our studies. We have never needed to terminate a study due to a lack of potential subjects.
Our Solutions
Interventional Trials
The InfantRisk Center utilizes facilities at the TTUHSC School of Medicine’s Clinical Research Unit facilities to conduct interventional studies. We offer full turnkey solutions, IND support, or can manage a trial to provide samples to you for analysis. We are available for any level of customization.
Average time to completion: 6-24 months
Non-Interventional Solution
The InfantRisk Center directly interacts with breastfeeding women taking an extensive range of medications. Our trials leverage this national network to quickly identify and enroll study subjects. Our non-interventional solutions include observational “registry”-type data collection as well as options to collect milk samples directly from women currently taking the medication of interest.
Average time to completion: 3-18 months
Pediatric Safety
Any design type can be augmented with long-term observation of pediatric events.
Average time to completion: 12+ months
Consultation
Our experts in lactation research can be consulted through our Sponsorship program for more lower-touch solutions.
Expert Experience
The InfantRisk Center is undoubtedly the pinnacle of medication-lactation research. However, we know our knowledge needs to be supported by experts in the disease state at hand. Through our university affiliation with TTUHSC, we have access to clinical experts across all medical specialties. We are well versed in working with CRO’s or directly with pharma.
Contact Us
For more information on conducting a clinical trial, fill out the form below.
If you are searching for breastfeeding drug information for yourself, do not use this form. Call our phone center advisors 8AM to 5PM, Monday - Friday at 1-806-352-2519. We will NOT answer personal questions here.